| Cresols are methyl substituted phenols at relative to the hydroxyl group,
ortho-,
meta-, and para-cresol. There are three structural isomers. The names of the
three compounds indicate which of the hydrogens on the benzene ring portion of
the molecule have been replaced. They are obtained from coal tar or petroleum.
Because the boiling points of these three compounds are nearly the same, a
separation of a mixture of the three into its pure components is impractical.
They are highly flammable and are soluble in water, ethanol, ether, acetone and
alkali hydroxides. The mixture of cresols obtained from coal tar is called
cresylic acid, an important technical product used as a disinfectant and in the
manufacture of resins and tricresyl phosphate. Cresylic acid also refs to the
mixture of phenols containing varying amounts of xylenols, cresols, and other
high-boiling fractions, but not more than 5 percent phenol. Commercial cresols
are prepared in a wide range of grades and purities to meet the user's
requirements. It is a liquid from clear to brown and is toxic to animals
including human. It is corrosive and is a more powerful disinfectant and
antiseptic than phenol. The primary use is for sterilizing as disinfectants and
deodorizers, and pesticides.Its solution is used as household cleaners as a
disinfectant. Creosote is a mixture of guaiacol, creosol and other phenolic
compounds obtained from wood tar (mainly beech) by distillation mostly between
203 and 220 C. It is a clear to yellowish, oily liquid; insoluble in water,
soluble in methanol, acetone. It is used as a wood preservative. It is used as
an external antiseptic, expectorant, gastric sedative, deodorant, and as an
antiseptic parasiticide veterinary use in the form of creosote carbonate. It is
used in the synthesis of pharmaceuticals and vanillin. Each cresols are used as
solvents or disinfectants and as useful as raw materials for various chemical
products icluding disinfectants, synthetic resins, fragrances, pigments, drugs,
antioxidants,
dyes, UV
absorbers, and pesticides. Cresols undergo electrophilic substitution reactions such
as chlorination, bromination, sulfonation and nitration at the vacant position.
They also undergo condensation reactions with aldehydes, ketones or dienes. O-cresol is a starting material for the synthesis of herbicides
such as 4,6-dinitro-o-cresol (DNOC) and 2-methyl-4-chlorophenoxyacetic acid
(MCPA). Meta-cresol is used in the manufacture of explosives. Meta and
para-cresol are used in phenol-formaldehyde resins and are converted to
tricresyl phosphate used as a plasticizer and gasoline additive and antioxidants
such as di-tert-butylcresols (BHT). Ortho- and para-cresols are used in the
production of lubricating oils and motor fuels.
Acetate is the ester that an organic group replaces a hydrogen atom in -OH group
of acetic acid through reaction (typically condensation) with alcohols.
Condensation is the reaction in which two molecules having -OH groups are joined
with eliminating a water molecule from their -OH groups. The term acetate is
also for the salt that one or more of the hydrogen atoms of acetic acid are
replaced by one or more cations of the base, resulting in a compound containing
the negative organic ion of CH3COO-. Organic acetates are good solvents for a
broad range of resins as they are miscible with almost all common organic
liquids. Due to their powerful solvency, high volatility and mild odor, acetates
are widely used in the manufacture and in the processing of paints, coatings,
adhesives, and printing industry. They have a very low solubility in water and
used as extraction solvents for fine chemicals particularly for certain
antibiotics. They are also used as components of aroma. They are used as
chemical intermediate to manufacture pharmaceuticals, synthetic flavorings,
cleaners, and other organic compounds.
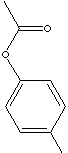
p-Cresyl
Acetate is used in formulating microbiocides and disinfectants.
It is used in perfumeries and flagrances.
|